Propelling Transportation Forward
Toward a Cleaner, Safer Future
A joyride. A cruise. A flight to your next vacation or a drive to see your family. Or just simply getting from point A to point B. Whatever the reason, there are few people who don’t appreciate a good ride. But aside from those times when something goes wrong – your car won’t start, or your plane is delayed, for example – most people take transportation, and the engineering that enables it, for granted.
It makes sense. Ground, sea, and air travel are well established and very reliable. To the naked eye, there’s not much left to do but add a few luxury options and call it complete.
But more and more, people across the globe are increasingly concerned about the state of transportation: specifically, our reliance on fossil fuels for propulsion and perhaps more importantly their effect on the environment.
According to the EPA’s web site, the burning of fossil fuels to power the transportation of people and goods is responsible for 31% of total carbon dioxide emissions in the US, the second-highest cause after only electricity. It was also the source of 26% of greenhouse gas emissions in the US in 2013. Globally, greenhouse gas emissions from transportation made up 14% of the total greenhouse gases in 2010.
“The most pressing need in energy production now – on the ground, in the air, or at sea – is not supply or cost of the fuel; it’s how to reduce CO2 emissions,” says Ronald C. Crane Professor Ahmed Ghoniem, director of the Center for Energy and Propulsion Research and the Reacting Gas Dynamics Lab. “With COP21, everyone agrees that we are producing lots of CO2 and not enough clean energy. We have our marching orders.”
Judging by the number of research projects related to these emissions, the urgency is abundantly clear, and many engineers foresee a major disruption on the horizon for the transportation industry as new solutions bubble up – some quickly and some gradually – to the consumer level.
In the MIT Department of Mechanical Engineering, faculty are researching solutions from almost every angle. Some, like Professor Wai Cheng, are focused on improving the efficiency of the engine; while others, like Professor Tomasz Wierzbicki and Professor Chryssostomos Chryssostomodis, are working on ways to improve implementation of battery-powered propulsion. Some are working on drag reduction to improve efficiency, some are finding new sources of renewable energy.
***********
For his part, Professor Ghoniem is focused on producing fuels that enable near-zero-carbon renewable sources to replace CO2-heavy fossil fuels. These include biofuels – fuels made from nonedible biomass and waste, such as grasses, woods, and fast growing plants – or hydrogen/synthetic gas from water/carbon dioxide mixtures.
Biofuels are an attractive option because they produce liquid fuels at the end of the process – which works out well in transportation because liquid fuels have the highest energy density, thereby allowing for longer drives without having to refuel. Synthetic gas, produced by reducing water or carbon dioxide emitted in power plants or other industrial processes, essentially “recycles” these products back into fuels using “renewable heat” such as solar heat. The syngas, as it’s commonly called, can be converted into liquid fuels for transportation applications using well established (Fischer Tropsch) processes.
“The implementation of electric cars is slow because they are still expensive,” says Professor Ghoniem, “and refueling requires an infrastructure that doesn’t currently exist at scale. So for now, we have to continue to rely on combustion-driven engines while still reducing CO2 emissions. One way to do that is to expand the use of fuels produced using renewable sources. The question is: How do you expand the production of these fuels?”
There are a few different processes that produce biofuels from “waste” biomass, but an efficient and economically sound one, says Professor Ghoniem, is a thermochemical process that turns it into synthetic gas first, then liquid fuel.
“It’s nearly a carbon-neutral process,” says Professor Ghoniem, “because the carbon from the material is turned into fuel; the fuel is burned and produces CO2; and the C02 is ingested back by the plants. It’s a closed cycle where the carbon moves from the plant to the fuel and then back to the plant.”
But it’s not as easy as it may sound. It is a complicated, multi-step process with variables that must be analyzed and balanced to achieve the optimal, most energy-rich outcome. But, if done optimally with the right knowledge, he says, it can be the most promising method of producing biofuel, as well as fuels from other waste.
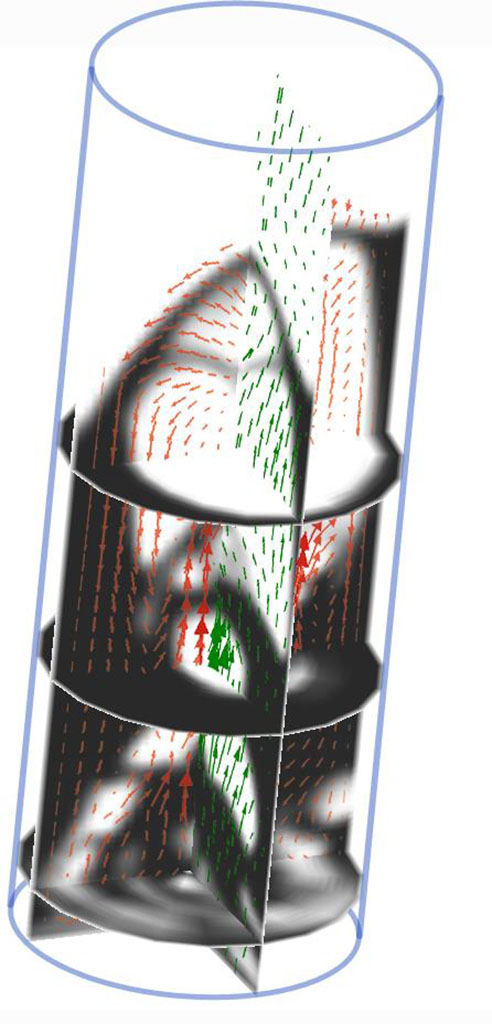
“It’s very difficult to measure things inside these systems because of the very hostile environment,” says Professor Ghoniem. “There are lots of things cooking and flying all over the place. People have been trying to operate these systems, but with mixed success. It is important to develop detailed predictive models that use supercomputers to make up for the lack of measurements to help designers and operators.”
Professor Ghoniem and his research team are developing such computational models to codify the fluid mechanics and chemistry of the process at many scales, to understand how to achieve the desired energy outcome for any types of biomass or waste, and to maximize the production of useful fuels and minimize the formation of undesirable products .
His group is also working on several innovative approaches to utilizing heat to produce fuel from water or carbon dioxide. This requires energy such as electricity or heat to break up the strong chemical bonds between oxygen and carbon monoxide or oxygen and hydrogen. “The process is like reverse combustion: We put heat in to get a chemical fuel out, instead of burning a fuel to get heat out as we do in combustion,” says Professor Ghoniem. “And to make it renewable, the heat source must be solar energy collected using large collectors/concentrators. This reduction (or dissociation) process produces fuel and oxygen, and both can be stored for later use.” Given the relatively low temperature of solar heat, enablers such as ceramic membranes are used to promote the processes.
*****************
Professor Tomasz Wierzbicki, the director of the Impact and Crashworthiness Lab, is also developing computational models to reduce CO2 emissions. His goal is to develop tools that can predict how materials, components, and structures will react to extreme loading, including everyday automobile crashes as well as other extreme conditions such as explosions.
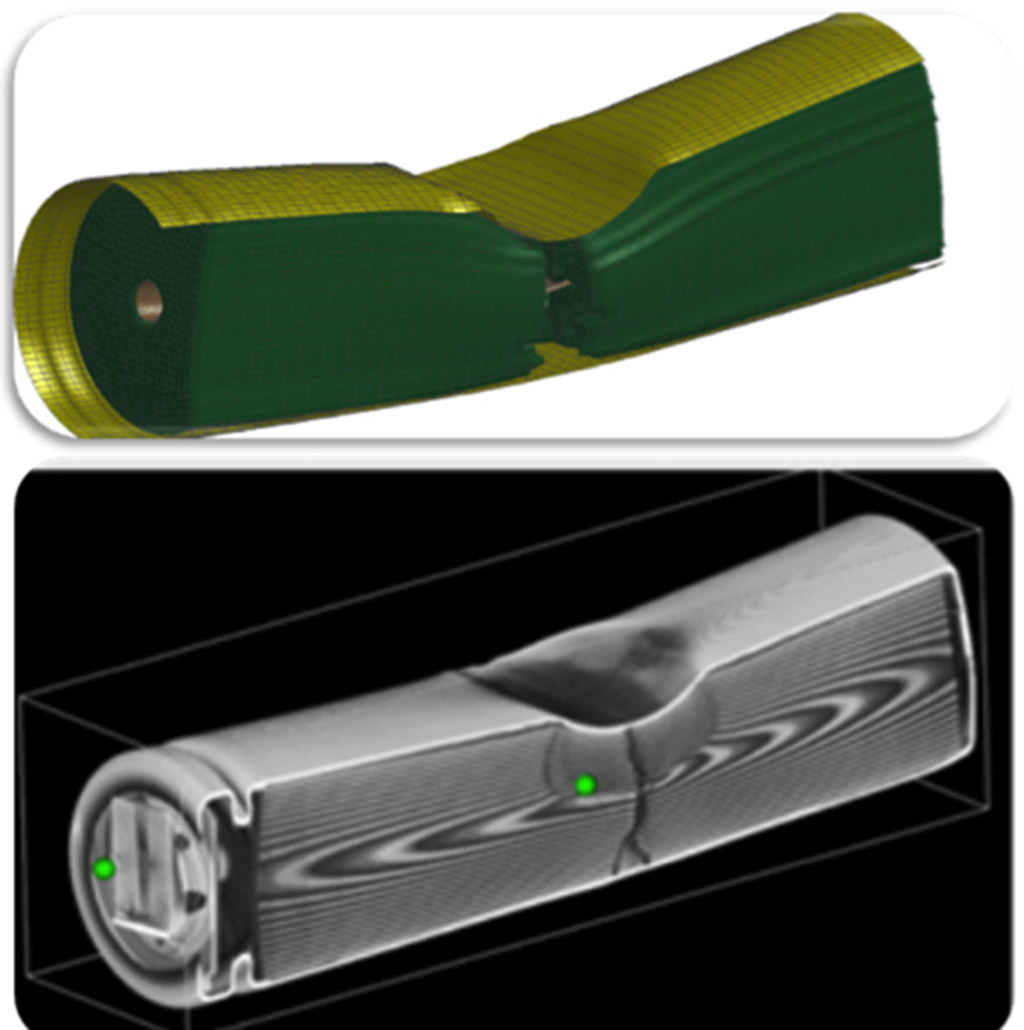
On the surface, that may not sound like it’s related to reducing carbon dioxide emissions, but his experience in modeling and predicting fractures also puts him in a good position to design a lighter material and structure, which can significantly reduce vehicle weight and in turn save energy, as well as to model the deformation and fracture of lithium-ion batteries in electric vehicles.
“I realized several years ago that we could help predict and prevent batteries from catching on fire and exploding in collisions involving electric cars,” says Professor Wierzbicki. “We knew that under a certain type of loading, such as hitting road debris or a side collision, an electric short circuit can develop inside a battery pack and cause a fire or explosion in a fraction of a second. So we wanted to understand the physics of that and provide the battery industry with the tools to predict what type of loading is safe and what type of battery is safe.”
Professor Wierzbicki is the coordinator of several consortia of industry leaders in automobile and battery manufacturing. Through the consortia, he shares his research with companies that want to find answers to questions about how automobiles and other vehicles will behave in certain impact scenarios, and how to design and manufacture vehicles that can provide better protection to the occupants and at the same time weigh less.
He is also working to develop another set of computational tools that will predict how to use additive-layer manufacturing to create structures as light as titanium and magnesium but much less expensive than those made from conventional stainless steel. “We are developing computational models now,” he says, “to prove that components made through the additive-layer manufacturing technique can be used as load-bearing elements and pieces with complicated shapes.”
But Professor Wierzbicki doesn’t ask his industry partners just to take his word on the reliability of his models. He and his research team have also designed and built their own testing machines along with specially designed specimens in order to validate their mathematical and computational models and prove that their predictions are accurate. In fact, the Impact and Crashworthiness Lab sets a high standard for itself by using wisely chosen constitutive models to predict material and structure behaviors with so far unattainable accuracy.
“We took part in the Sandia Challenge One and Two,” he says, “which invited labs around the world to make blind predictions about fracture. The Sandia provided the labs with some limited information about a material and asked participants to predict what would happen next under a prescribed loading. The Sandia researchers conducted their own physical test and later on compared it to each lab’s predictions to see how accurate they were.”
In the Sandia Challenge Two, Professor Wierzbicki’s lab came in first ahead of 13 competing labs.
“Looking into the future,” he says, “we are confident that we can predict fracture under impact and crash loads of more complex materials and structural systems, such as welded pipes for risers and linepipes; fragment-impact containment structures of exploding airplane turbofan engines; and burst containment of turbo chargers of racing and passenger cars, as well as low- and high-cycle fatigue loads. These ambitious tasks will be accomplished in close cooperation with top European universities and worldwide industries.”
***********
Professor Wai Cheng has been a member of the Sloan Automotive Lab since 1980, and has been serving as the Lab’s director since 2008. He and his team of researchers are focused primarily on improving engine efficiency to reduce carbon dioxide emissions. It’s a much more delicate balance of requirements and constraints than it may seem on the surface, and what’s interesting, he says, is that in many ways he’s solving problems he’s already solved once before – but this time it’s for different reasons.
“A lot of the old topics become new topics again,” he says.
For example, one way to decrease CO2 emissions in automobiles is to make the engines smaller so that they operate more efficiently in situations such as cruising – but by doing so, you also decrease the power output when you need it for power-heavy situations such as climbing hills. To make up for that, you add a turbo charger, which boosts more air into the engine, and thus, burns more fuel and produces more energy.
But then suddenly you are presented with a problem that has already been solved, well, many times actually, going back to the 1930s: knocking, which is essentially a mini-explosion in the engine caused by the air and the fuel igniting very quickly. The increased temperature and pressure of the turbo-charged engine induces knocking once again.
Cheng and his team are focused on researching variables that induce or reduce knocking – such as the fuel effects (including the use of alcohols and other alternative fuels) and the way it is introduced into the engine – in context of the modern constraints imposed by small engines and turbo-chargers.
At the same time, he is also working on addressing another old problem: particulate emissions. Although they were a problem in the 1970s and 1980s in engines generally, spark ignition emissions were never much of a concern because they were so low. Today, however, scientists understand that they contain small particles that can cause significant health effects despite a low mass emission. To make matters worse, direct fuel injection, which is a method used to suppress knock, leaves a residual liquid fuel on the cylinder walls that is a major source of particulate emissions.
Cheng and his team are researching ways to minimize these startup emissions. The catalytic converter was a very effective invention that reduces tailpipe emissions by several orders of magnitude – but only after the catalyst reaches a certain temperature (referred to as “light-off”). For a typical trip, more than 90 percent of the emissions come from the first 10 or 20 seconds of operation while the catalyst is still warming up. Professor Cheng’s team is working on engine strategies to reduce both the catalyst light-off time and the level of emissions during that time.
The delicate domino effect manifests thusly: Increase the efficiency, lose power. Maintain power, introduce knocking. Fix knocking, decrease efficiency, increase emissions.
“Everything interacts,” says Professor Cheng. “The hope is that you can push on all fronts so that you get a better car and a better engine, but it is always a balancing act.”
**************
As challenging as it is to engineer within seemingly contradictory constraints, it is even more difficult deep underwater, where there are a whole slew of additional issues. MIT Sea Grant graduate students and researchers, led by Professor Chryssostomos Chryssostomidis, want to be able to command autonomous underwater vehicles (AUVs) from their office, sending them down into the ocean to collect data, predict hot spots, deploy sensors, identify environmental issues, and send back information to the on-land operator.
But underwater, refueling is currently impossible without bringing AUVs back to the surface, which is a costly and time-consuming process. And along with this limited energy storage also comes a limited radius for data collection – and therefore limited data.
“Imagine if we had ‘gas stations’ in the ocean,” says Professor Chryssostomidis, “and I could take my vehicle there to refuel and then just continue on with my work. Right now, I’m forced to work within a radius. No matter how efficient I make my propulsion, I will always run out of juice at some point. There is no way I can invent the perpetual machine.”
One way Professor Chryssostomodis is solving for that problem is by developing wireless underwater battery recharging. Professor Chathan Cooke, Principal Research Engineer at MIT Research Laboratory of Electronics, and Michael Defilippo, Research Engineer at MIT Sea Grant College Program, have conducted research that shows that a four-coil wireless recharging station using low series resistance minimizes energy losses within the circuit and maximizes the transferred power. The high resonant factor of this system can make up for a minor misalignment or large separation between the transmitter and receiver coils, enabling more than 90% efficiency of power transfer. Now, Michael Defilippo is working on how to overcome the effects of saltwater on the system.
“The related technology that goes very much into the heart of underwater autonomous transportation is that of electric cars,” says Professor Chryssostomodis, “because electric cars could also recharge the same way. But trying to accomplish that same thing in the ocean is even more complicated because the salt environment causes a lot of parasitic effects.”
The electric ship is another mode of transportation that Professor Chryssostomodis and his research group at MIT Sea Grant are working on. The power demands of a military ship are forever increasing, especially as power-heavy electrical modern weapons like lasers and ray guns are added. Meanwhile, the ships still need to move ahead at moderately high speeds, requiring power at the cube of the speed. Traditionally, ships have been powered mechanically, but Professor Chryssostomidis’s idea is to make everything electrical.
“The power demands we have are just phenomenal,” he says. “The Empire State Building requires 10 megawatts. The ship we are designing right now requires 100 megawatts. And it’s similar for cruise ships as well. The ship is 150 meters long, so it becomes important that the power cables are precisely positioned. ”
Researcher Julie Chafant and Postdoctoral Associate Hessam Babaee are developing models to help optimize the design of the power cables using a variety of criteria, not least of which is the cooling system for all this power transfer, which generates an incredible amount of heat.
*******************
Many of us do appreciate our modes of transportation, as well as the engineering that enables them and perhaps because of that we expect so many things: quickness, reliability, comfort, exhilaration, safety. And yet we know we need to do this in a clean, responsible way that doesn’t cause more damage to our environment. As it stands, our desires and our needs are in conflict, and it’s up to us to find a way to reconcile them.