Study finds potential in brackish groundwater desalination
New research suggests there’s a large untapped resource for many of the increasingly water-limited regions of the U.S. and around the world: brackish groundwater, which, in theory at least, would require much less energy to desalinate than seawater.
The amount of brackish groundwater in the United States is about 800 times greater than the total amount of groundwater withdrawn nationwide for all uses, according to a U.S. Geological Survey study. So, using even a fraction of that resource could dramatically improve the prospects for water-starved communities, especially as potable water becomes more scarce, as climate change models predict. While about a fifth of the nation’s water supply comes from fresh groundwater, less than 1 percent currently comes from brackish groundwater.
The findings, based on analysis of a massive dataset of water samples from around the country, appears in the journal Water Research, in a paper by Yvana Ahdab SM ’17, an MIT graduate student, with John Lienhard, the Abdul Latif Jameel Professor of Water and Food, and three others.
“Until recently, brackish groundwater has been a largely overlooked resource, despite its potential to relieve the mounting pressure on fresh water supplies,” Ahdab says.
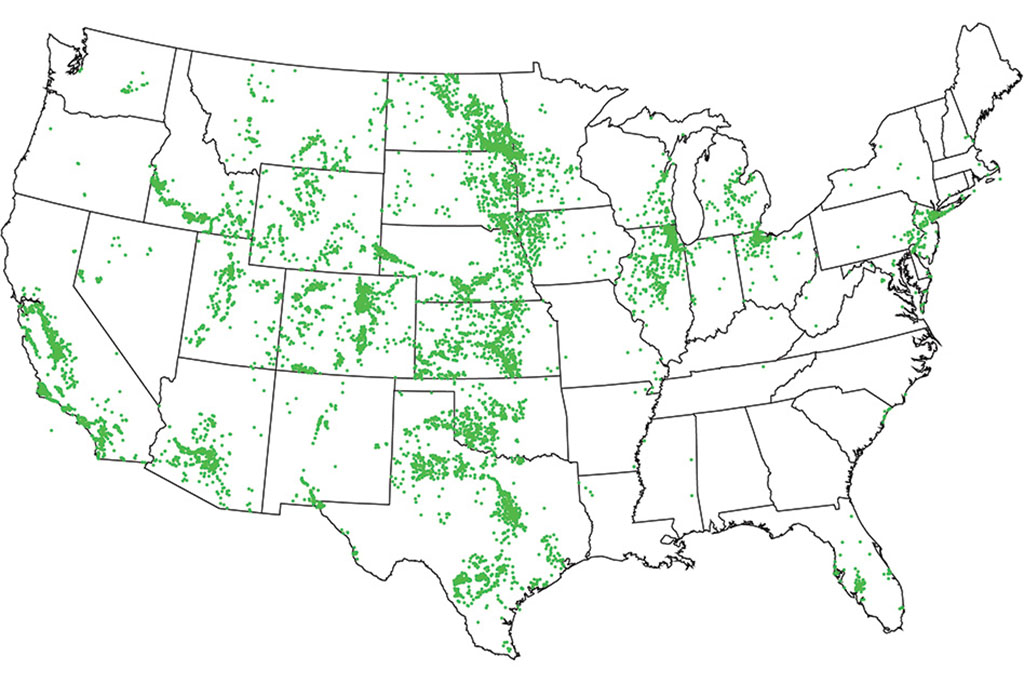
The project started when Lienhard met a USGS scientist at a conference in 2015 and learned about a collection of more than 100,000 groundwater samples from wells, which had just been taken by that agency. “I was fascinated, and I wondered what we know about how the composition of groundwater affects the desalination process,” he recalls. He discussed this question with Ahdab, who quickly found that while seawater composition around the world had been well-studied, no comparable studies of groundwater had been carried out. (The composition of groundwater varies much more widely, in both saltiness and the concentration of other substances such as calcium and sulfate.) The USGS dataset was just publicly released last year, but the MIT team was granted early access to begin working on it in collaboration with USGS scientists.
While seawater around the world has essentially the same composition with only minor variations, Ahdab explains, “groundwater is much less salty, about a tenth as much, and the set of ions [charged atoms or molecules] is completely different at every different well.”
Because of the lower concentration of salt in brackish water, some technologies such as distillation, which can make sense for seawater in some locations, would be very energy-inefficient for desalinating brackish water, Lienhard says. “Reverse osmosis (RO) or electrodialysis (ED) are the two technologies of choice, and they are the two that are most commonly used.”
Because seawater is the main feedstock for existing desalination systems, the technology has mostly been developed and optimized for seawater’s composition. Since brackish water is so different, Lienhard says, “The question we’re interested in is: How much room is there for improvement?” The present study doesn’t examine the specific technologies that could be used, which will be addressed in further research, but it does look at the theoretical limits of how much it would be possible to reduce the energy required to desalinate brackish water that has a particular composition.
“We found that the compositions fall into five classes,” Ahdab says. “Some are dominated by sodium chloride, sodium bicarbonate, or sodium sulfate, while others are dominated by calcium bicarbonate or calcium sulfate. They require very different approaches, in terms of the kind of pretreatment you need.” The new analysis, which identifies the specific compositions of the water down to the level of individual wells, “will help to guide the development of new systems,” she says.
The team found that groundwaters dominated by sodium require much more energy to desalinate than those dominated by calcium, as do those dominated by chloride compared to those dominated by sulfate. That information, coupled with the maps of brackish water composition across the country, can help guide plans to develop new water resources, Ahdab says.
In addition, the team correlated the location of potentially usable brackish groundwater sources with the regions that are experiencing the greatest stresses on their water supplies. The results point to specific locations that have the most promising combination of a high need for water and high availability of treatable brackish water, including parts of southern California, Arizona, and areas of the upper Midwest. “There’s an enormous amount of brackish groundwater which can be desalinated for fairly low amounts of energy,” Lienhard says. But more work needs to be done to translate that low need for energy into practical desalination systems that actually have correspondingly low costs, he says.
While the most complete dataset was based on U.S. wells, the team also examined other, less complete sets of samples from a number of other countries, and found that the U.S. trends in composition and desalination energy of treatable brackish water are likely to be replicated in most parts of the world.
One big advantage of desalinating brackish groundwater rather than seawater, the researchers say, is the recovery rate: While seawater desalination typically yields half its volume in fresh water, desalination of brackish water can yield as much as 90 percent fresh water. That also makes the last part of the process much easier. Because the brine left over from brackish desalination is much more concentrated, there’s proportionally much less of it, which simplifies disposal. In fact, Lienhard says, it may even be possible to get the water out altogether, leaving just solid salt which would be even easier to dispose of (or find uses for).
Prakash Rao, a researcher at Lawrence Berkeley National Laboratory, who was not involved in this research, says “water shortages in the U.S. are imminent, and the scientific community is needed to address this challenge. Greater utilization of alternate water supplies, like brackish water, to meet growing water demand in the U.S. is likely. But, currently brackish water is seldom used in the U.S. This creates an urgent need to better understand how best to use our vast brackish water resources. This paper makes an important contribution to this end.”
Rao adds that “Using the approach developed in this paper, water agencies, municipal water authorities, and desalination system designers can make more informed decisions.”
And Rick Stover, director of the International Desalination Association, who also was not involved in this study, says “In my view, the major significance of this work is how comprehensive its geographical reach and its compositional analysis are. It represents a roadmap for brackish desalination, especially in the U.S.”
Stover concludes that “Brackish desalination will likely become a major source of water supply for inland locations around the world. The information presented in this paper provides essential information to guide that development. It’s both significant and timely.”
The team included former MIT postdoc Gregory Thiel PhD ’15, and J.K. Böhlke and Jennifer Stanton of the USGS. The work was supported by the National Science Foundation, the U.S. Geological Survey Water Availability and Use Science Program and U.S. Geological Survey National Research Program.