Ingestible electronic device detects breathing depression in patients
Diagnosing sleep disorders such as sleep apnea usually requires a patient to spend the night in a sleep lab, hooked up to a variety of sensors and monitors. Researchers from MIT, Celero Systems, and West Virginia University hope to make that process less intrusive, using an ingestible capsule they developed that can monitor vital signs from within the patient’s GI tract.
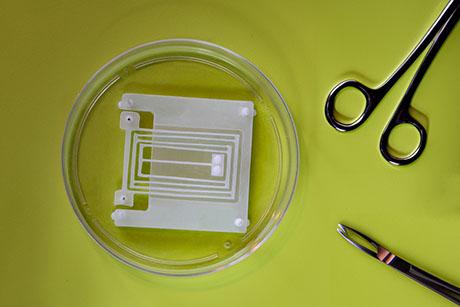
The capsule, which is about the size of a multivitamin, uses an accelerometer to measure the patient’s breathing rate and heart rate. In addition to diagnosing sleep apnea, the device could also be useful for detecting opioid overdoses in people at high risk, the researchers say.
“It’s an exciting intervention to help people be diagnosed and then receive the appropriate treatment if they suffer from obstructive sleep apnea,” says Giovanni Traverso, an associate professor of mechanical engineering at MIT and a gastroenterologist at Brigham and Women’s Hospital. “The device also has the potential for early detection of changes in respiratory status, whether it’s a result of opiates or other conditions that could be monitored, like asthma or chronic obstructive pulmonary disease (COPD).”
In a study of 10 human volunteers, the researchers showed that the capsule can be used to monitor vital signs and to detect sleep apnea episodes, which occur when the patient repeatedly stops and starts breathing during sleep. The patients did not show any adverse effects from the capsule, which passed harmlessly through the digestive tract.
Traverso is one of the senior authors of the study, along with Robert Langer, an MIT Institute Professor and member of MIT’s Koch Institute for Integrative Cancer Research; Victor Finomore, director of the Human Performance and Applied Neuroscience Research Center at the West Virginia University School of Medicine; and Ali Rezai, director of the Rockefeller Neuroscience Institute at the West Virginia University School of Medicine. The paper appears today in the journal Device.
Vital sign measurements
Over the past decade, Traverso and Langer have developed a range of ingestible sensors that could be used to monitor vital signs and diagnose disorders of the GI tract, such as gastrointestinal slowdown and inflammatory bowel diseases.
This new study focused on measuring vital signs, using a capsule developed by Celero Systems that includes an accelerometer that detects slight movements generated by the beating of the heart and the expansion of the lungs. The capsule also contains two small batteries and a wireless antenna that transmits data to an external device such as a laptop.
In tests in an animal model, the researchers found that this capsule could accurately measure breathing rate and heart rate. In one experiment, they showed that the sensor could detect the depression of breathing rate that resulted from a large dose of fentanyl, an opioid drug.
Building on those results, the researchers decided to further test the capsule in a clinical trial at the West Virginia University Rockefeller Neuroscience Institute. Ten patients who enrolled in the study were monitored using the ingestible capsule, and these patients were also connected to the sensors typically used to monitor sleep, so the researchers could compare measurements from both types of sensors.
The researchers found that their ingestible sensor was able to accurately measure both breathing rate and heart rate, and it also detected a sleep apnea episode that one of the patients experienced.
“What we were able to show is that using the capsule, we could capture data that matched what the traditional transdermal sensors would capture,” Traverso says. “We also observed that the capsule could detect apnea, and that was confirmed with standard monitoring systems that are available in the sleep lab.”
In this study, the researchers monitored signals emitted by the capsule while it was in the stomach, but in a previous study, they showed that vital signs can also be measured from other parts of the GI tract.
“The stomach generally offers some of the best signals, mainly because it’s close to the heart and the lungs, but we know that we can also sense them elsewhere,” Traverso says.
None of the patients reported any discomfort or harm from the capsule. Radiographic imaging performed 14 days after the capsules were ingested revealed that all of them had passed through the patients’ bodies. The research team’s previous work has shown that objects of similar size usually move through the digestive tract in a little more than a day.
Close monitoring
The researchers envision that this kind of sensor could be used to diagnose sleep apnea in a less intrusive way than the skin-based sensors that are now used. It could also be used to monitor patients when they begin treatment for apnea, to make sure that the treatments are effective.
Celero Systems, a company founded by Traverso, Langer, Jeremy Ruskin, a professor of medicine at Harvard Medical School, and Benjamin Pless, now CEO of the company, is now working on sensors that could be used to detect sleep apnea or opioid overdose.
“We know that people who have had an overdose are at higher risk of recurrence, so those individuals could be monitored more closely so that in the event of another overdose, someone could help them,” Traverso says.
In future work, the researchers hope to incorporate an overdose reversal agent such as nalmefene into the device, so that drug release would be triggered when the person’s breathing rate slowed or stopped. They are also working on strategies to lengthen the amount of time that the capsules could remain in the stomach.
The research was funded by the Karl van Tassel Career Professorship, MIT’s Department of Mechanical Engineering, and Celero Systems.
Authors of the paper also include Pless, James Mahoney, Justin Kupec, Robert Stansbury, Daniel Bacher, Shannon Schuetz, and Alison Hayward.