2.013: Engineering Systems Design
Breakthrough fuel alternative may allow electric vehicle charging on demand.
With the unstable cost of petroleum perpetually threatening to shoot upwards, and its potentially devastating effects on the environment waiting anxiously in the wings, many people are hopeful that electric vehicles will provide a cleaner, cheaper option to diesel- or gasoline-powered vehicles.
But there are still several problems that engineers are working to solve before this hope can become a reality, the most major of which is recharging. For many consumers, it’s currently a deal-breaker: You have to recharge too often and it takes too long. And that’s assuming that recharging stations exist at the necessary distances in the first place.
Undergraduate students in Professor Doug Hart’s 2.013/2.014: Engineering Systems Design/Development capstone sequence are developing a promising solution: aluminum fuel.
“Relative to batteries,” says one student CEO, Nicholas Fine, in this year’s class, “the amount of energy you can store with aluminum fuel relative to the amount of mass you can carry with you is significantly higher – which means your mission duration is significantly longer.”
2.013/2.014 started back in 2011 in response to a challenge by MIT Lincoln Laboratories: Develop an energy source for underwater systems that can increase endurance tenfold.
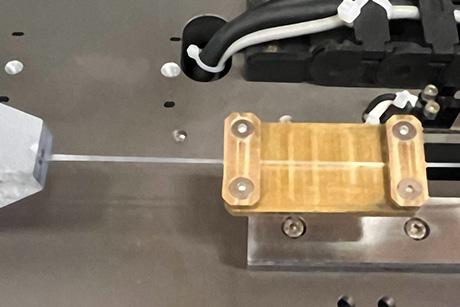
The first year’s class of students – who worked together under a CEO like a real-world design team – discovered that aluminum can react with seawater in a particular way to generate hydrogen gas. The resulting fuel is created by stripping the passivation layer from aluminum using a gallium-based eutectic, then reacting the aluminum with water to generate hydrogen gas and heat.
When this reaction occurs, student David D’Achiardi explains, about half the energy goes into the heat and half goes into the hydrogen. Since hydrogen can be converted to electricity at higher efficiencies than fossil fuels using a fuel cell, and since aluminum is nearly four times as energy dense as diesel, aluminum provides a very safe and efficient energy source without the need to store compressed hydrogen.
“But,” says D’Achiardi, one of the class’s CEOs, “if you are able to recover a portion of the heat energy, your fuel consumption is dramatically decreased, and that is where the fuel becomes extremely interesting as a very high-energy-density fuel.”
The discovery, originally made by Jonathan Slocum when he was a student in 2.013, led to several progressive underwater autonomous vehicle (UAV) power systems in subsequent years of class that could monitor the oceans at depth for weeks at a time.
This year’s 2.013 class is pursuing other possible applications for the technology, including electric cars. Students have organized themselves into four separate teams, each with their own CEO and their own potential application.
One team, led by Laura Jarin-Lipschitz, is designing a way to use the aluminum fuel to power a submersible power station that rises to the surface to recharge drones for search and rescue missions; another, led by Alexander Klein, is looking at how to automate the production of aluminum fuel and lower its cost; yet another is designing a portable emergency charging device for military situations, led by Fine.
The fourth team, led by D’Achiardi, is designing a way to extend the range of electric cars using an onboard aluminum-fueled generator.
“We want to show that this technology could be comparable, competitive, or possibly even surpass what a gas or diesel vehicle would look like in terms of cost, volume of the fuel, and mass of the fuel,” says D’Achiardi. “These are all metrics we are using to shift and propose designs.”
His team is working to design a heat-recovery system to take advantage of the heat energy generated, while also trying to devise a way to draw power from the fuel into the battery very quickly, so that electric vehicles can get the same range as fossil-fueled vehicles – all without modifying current electric vehicles.
“It’s tricky,” he says, “because we are trying to generate power inside the vehicle, using this clean technology.”
Many of the students – some of whom are taking 2.013 for the second time now – plan to continue on with the development of their designs in the spring with sister class 2.014.