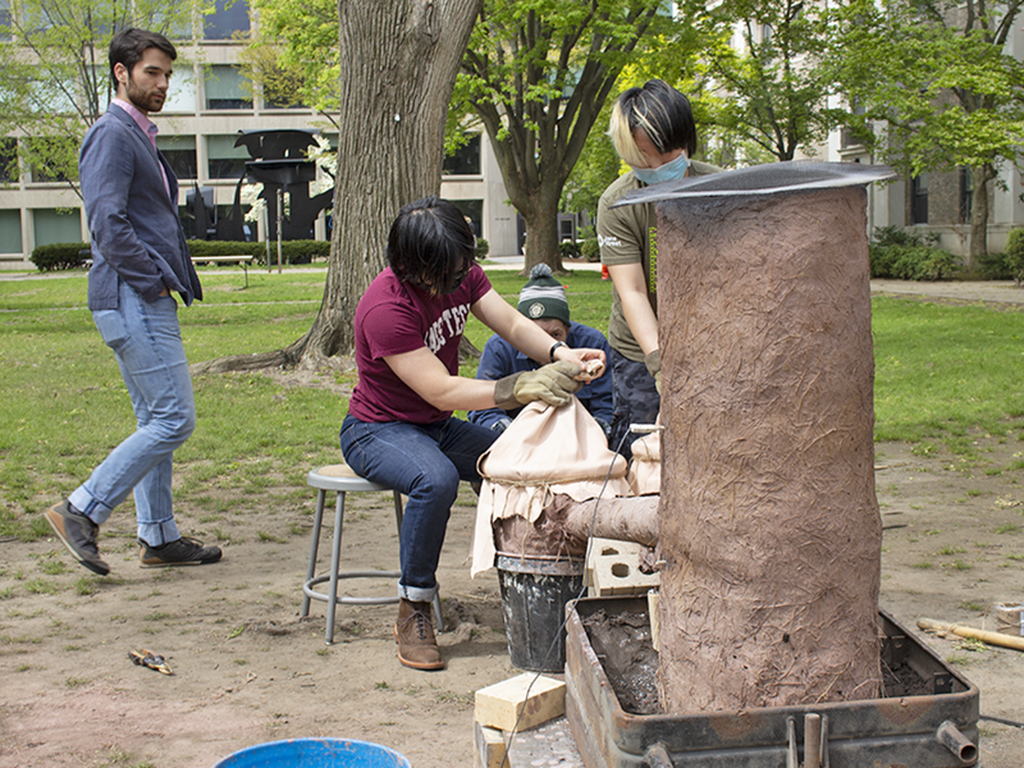Ancient African smelting technique sparks anew at MIT
The plumes of smoke that rose from East Campus one sunny May day could easily have been mistaken for a barbecue taking place in the courtyard. And indeed, burgers were on the grill. But the smoke was coming from a much rarer sight on MIT’s campus — or, in fact, anywhere outside West Africa: a mud-and-straw furnace for smelting iron.
For two months, students in class 3.094 (Materials in Human Experience) had been building the furnace, or bloomery, in the traditional style of the Mossi people of Burkina Faso. The furnace was a five-foot-high cylinder. From the top end came dancing orange flames and billowing gray smoke; from an opening on the lower third of the cylinder extended two tubes, each into a large pot topped with goatskin and pumped by students and volunteers in T-shirts and polo shirts.
“The idea of this class in general is to teach people about materials through archeological materials,” says Mike Tarkanian, senior lecturer in the Department of Materials Science and Engineering. The first half of the class, taught by associate professor of civil and environmental engineering Admir Masic, focused on Roman concrete, the material used to construct ancient landmarks such as the Pantheon in Rome.
In the second half, Tarkanian and lecturer James Hunter taught about the long history of smelting iron in Africa, how iron helped fight people fight wars and till fields, and some of the current problems with iron and steel production — it’s one of the biggest producers of carbon dioxide. “And today they’re learning about how difficult this thousands-year-old process actually is.”
Old practice, new hands
In class, held in MIT’s foundry, students got practice pumping the bellows. They’d sit in front of two pots, grabbing a folded piece of goatskin in each hand and then making vigorous up and down motions, alternating between left and right. Pulling up unseals a flap in the goatskin, taking in air; pushing down closes the flap, expelling the air from the tubes and into the furnace, feeding the flames. But instead of a fire at the other end of the bellows was a plastic garbage bag, to give a sense of how much airflow students could produce. For the actual smelting, in the courtyard, students planned on pumping for 15-minute intervals.
“I came in very confident that I was going to be able to do it for 15 minutes straight because I did it for one minute in class. And it was incredibly exhausting,” says 3.094 student Sreya Vangara '22, an electrical engineering and mechanical engineering major who graduated in May. She spoke above the “thwump, thwump” sound of the bellows in the background. “When we have a real-fire at the other end of the bellows rather than an inflatable bag, the heat changes your ability to work, because all of a sudden, when you open up the flap, your knuckles get scorched.”
The project was inspired by a 2005 documentary video describing the traditional practice of smelting iron from iron ore in Burkina Faso. The video follows a family of smiths reviving the 2,000-year-old method, which had not been done for decades. It follows the men through every painstaking stage of the process, from making charcoal from the burnt remains of wood to mining iron ore — the rocks and minerals iron can be extracted from — to digging up clay, moistening it with water, and adding fresh hay to shape the furnace.
Tarkanian and the class used the video as a sort of manual to build the furnace. It’s made of terracotta clay bought from art supply store Blick Art Materials in Boston and grass harvested from Waltham, Massachusetts.
“We built the closest facsimile that we could to what we saw them doing in Burkina Faso,” Tarkanian says. “We had to make some guesses, but it looks pretty good.”
All the materials needed for smelting — charcoal, iron ore, and sand — were bought from stores and suppliers. The process works this way: a fire is lit in the furnace opening at the bottom, and charcoal and iron ore are layered into the top of the tube. As the charcoal burns, it reduces the ore to iron. Sand is added as flux — a material that melts into a fluid. “It basically reacts with the iron and can bring the iron down to the bottom,” Tarkanian says.
“Slag and junk”
Much later — the process documented in the video took 10 hours, while the MIT team smelted for about eight — the ore is pulled out from the furnace, “kind of like a sponge,” Tarkanian says, along with slag, a byproduct of iron smelting, which is thrown out. The metal is then banged and smacked into shape with hammers. Then that refined block of metal can be forged into tools or grates or whatever else. In the video, smiths turned the iron they got from the smelting into hand-held garden hoes.
Tarkanian and the class used 60 pounds of iron ore throughout the day. The output, in the best-case scenario, would be about 18 to 20 pounds of iron. The result was much less: just a few pellets, or prills, as they’re known in metallurgy.
“The rest was just slag and junk,” or glass and other non-metal materials, Tarkanian says. The likely reason was the fire didn’t reach the optimum temperature for a long-enough time to convert the ore into iron.
But the objective of the project wasn’t, strictly, to produce iron. Neither was it to seek out a more environmentally friendly smelting process than current ones; the Burkina Faso-inspired method generated carbon dioxide emissions that are the same or worse, because of the “excess” of charcoal burned, Tarkanian says — about 20 kilograms, or 44 pounds. The objective instead was to gain experience and insight into how an ancient society transformed natural materials into objects of material culture.
“Looking at material from an archeological point of view, you’re able to better dissect the underlying science, because the people who originally came up with this technology started with nothing, no scientific knowledge. And they worked their way up into developing the system,” Vangara says. “So every step of the way they were discovering something new. They were experimenting and realizing, ‘OK, we actually need to start with this ratio of charcoal.’ Or, ‘This is the temperature we need to bring it up to. Why?’”
Vin Armelin, a rising senior in chemistry and biology and biological engineering, took 3.094 as part of his humanities requirement. For him, both the class and the smelting project offered a fresh perspective.
“In science, everything you do needs to be incredibly precise. So you’re adding exactly a certain percentage, or there’s exactly this much volume,” Armelin says.
Making the clay-and-grass furnace was much different. When Armelin asked his instructors what measure of grass should be used, “they’re like, ‘Yeah, just keep shoving it in until it won't take anymore,’” he says. “It helps give some idea of how precise you really need to be depending on what you’re trying to achieve — and a different, more building-focused perspective that I didn't really have before.”