Faculty Research: Professor Roger Kamm
Not many people have watched as a single tumor cell sneaks its way through a blood vessel wall and out the other side. Professor Roger Kamm is one of the few who has.
Visualizing Sneaky Tumor Cells
 |
Professor Roger Kamm and PhD candidate Ioannis Zervantonakis. |
| Photo Credit: Tony Pulsone |
by Alissa Mallinson
Not many people have watched as a single tumor cell sneaks its way through a blood vessel wall and out the other side.
But Roger Kamm, the Cecil and Ida Green Distinguished Professor of Biological and Mechanical Engineering, and his doctoral student Ioannis Zervantonakis are two of the few who have. They are studying the mechanics of metastasis, the process of cancer cell migration from one location in the body to another and the cause of more than 90% of cancer deaths.
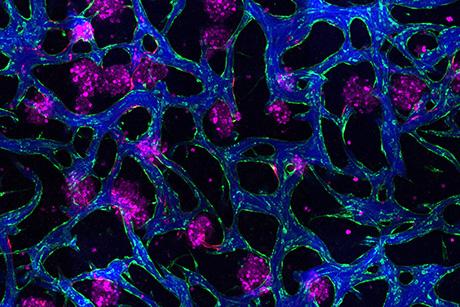
Professor Kamm has studied several aspects of metastasis over a period of several years using a 3D in vitro microfluidic device developed in his lab, including a recent study on the effect of flow on tumor cell migration. This time, the team used a device designed and developed by Zervantonakis to look at the importance of signaling between tumor cells and macrophages, a type of white blood cell with a versatile role in the immune system. They found that when macrophages are absent, it is extremely rare for tumor cells to migrate across the cell layer that lines the blood vessels (a process called intravasation), but, conversely, when they are present, the rate of entry increases significantly.
“It’s very exciting to have developed one of the first systems to image this process in real time and be able to characterize transport across the endothelial barrier,” says Zervantonakis. “Previous designs in our group allowed for endothelial cell and tumor cell cultures, but because intravasation is so rare — only about 5% of cells achieve it — it was hard to capture it in the two to four regions we had. So I developed a new assay based on a microfluidic system designed in collaboration with post-doctoral associate Waleed Farahat and PhD candidate Levi Wood from Professor Harry Asada’s group. It allowed us to observe at least 200 cells, using 40 regions of observation.”
Zervantonakis also included in his design the ability to measure permeability while quantifying tumor cell invasion by using fluorescently labeled proteins and incorporating a y-junction to balance the fluid flow. Combined with the significant increase in number of regions and the integration of the gel matrix that enables 3D culture, Kamm and Zervantonakis studied tumor cell intravasation as no one has ever done before.
This unprecedented ability to visualize intravasation confirmed several theories about the intricate mechanics taking place – which they published this year in the journal Proceedings of the National Academy of Sciences.*
First, Kamm and Zervantonakis confirmed the importance of the presence of bacteria-fighting macrophages. Because a lonely few tumor cells can cross through the endothelium even without the help of macrophages, the duo determined that their presence wasn’t essential; but they identified a strong positive correlation.
Second, they detected cell-to-cell communication between both invasive tumor cells and normally protective macrophages, and, subsequently, between traitor macrophages and the blood vessel. The result is increased leakiness of the vessels and tumor cells that can enter them.
Additional experiments – which involved replacing macrophages with a tumor necrosis factor called TNF-α, which is known to increase vessel leakiness – helped to determine the mechanisms more specifically and confirm a causal relationship. By performing these control experiments, they determined that macrophages alone were instrumental in increasing the blood vessel diffusive permeability and were at least causal in an increase in the migration of the tumor cells, if not the primary trigger.
“Experiments that included macrophages alone and then only TNF-α – a factor that macrophages secrete – both resulted in increased intravasation,” says Zervantonakis. “Because we have this method of measuring permeability in real time, we saw over the course of eight hours that the endothelium becomes leaky. We proved that when we make the endothelium leaky, more intravasation occurs. When we decrease the leakiness by blocking antibodies, less intravasation occurs.”
Kamm and Zervantonakis’s work provided support for the theory of John S. Condeelis, from the Albert Einstein Medical Center in New York, whose studies on mice had suggested that macrophages were important in this migration process. Being limited to live cell models only, Condeelis contacted Kamm after hearing news of his 3D in vitro microfluidic assay, which allows not only imaging of the cell movement but also the ability to consequently infer this crucial element of signaling between cells, something that no one had been able to incorporate into their in vitro assays. Unlike other systems, Kamm and Zervantonakis’s device enables them to visualize the cells at very precise locations within the system, even allowing them to discover that macrophages do not need to be in physical contact with the tumor cells but simply close enough to communicate with them.
“This is good news not only for the fundamental understanding of how metastasis occurs, but also because intact endothelial vessels block intravasation,” says Zervantonakis. “Although leaky vessels can be a positive thing when you want to distribute cancer-fighting drugs, tumor cells may utilize these same leaky vessels to enter the blood stream and travel to another part of the body. Additional studies are needed to investigate ways to target the role macrophages have in making blood vessels leaky.”
*I.K. Zervantonakis, S. K. Alford-Hughes, J. L. Charest, F. B. Gertler, J. C. Condeelis and R. D. Kamm (2012). “Three-dimensional microfluidic tumor-vascular interface model: Tumor cell intravasation and endothelial barrier function.” PNAS, 109 (34), 13515-13520]. Read the published paper here: http://bit.ly/UkPzTk