Faculty Research: Professor Rohit Karnik
In a New Microchip, Cells Separate by Rolling Away
 |
 |
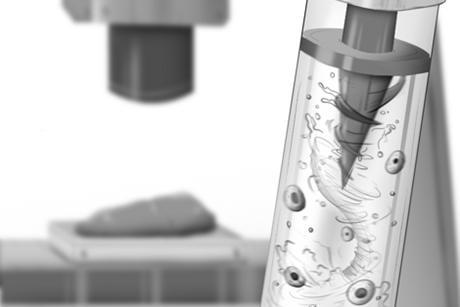
Associate Professor Rohit Karnik in his lab. | Karnik’s new microfluidic device isolates target cells (in pink) from the rest of the flow by getting them to stick weakly to the device’s ridges then roll through trenches and into a collection chamber. |
| Photo Credits: MIT News Office | |
by Jennifer Chu, MIT News Office
Cell rolling is a common mechanism cells use to navigate through the body. During inflammation, for example, the endothelial cells that line blood vessels present certain molecules that attract white blood cells just enough to divert them from the rest of the vessel’s cellular traffic. White blood cells then roll along the vessel wall, slowing down to help in the healing of inflamed areas.
Researchers at MIT and Brigham and Women’s Hospital – including MIT Professor Rohit Karnik, postdoc Sung Young Choi of MIT, and Jeffrey Karp, Associate Professor at Harvard Medical School and co-director of the Center for Regenerative Therapeutics at Brigham and Women’s – have now designed a cell-sorting microchip that takes advantage of this natural cell-rolling mechanism. The device takes in mixtures of cells, which flow through tiny channels coated with sticky molecules. Cells with specific receptors bind weakly to these molecules, rolling away from the rest of the flow and out into a separate receptacle.
The cell sorters, about the size of postage stamps, may be fabricated and stacked one on top of another to sift out many cells at once – an advantage for scientists who want to isolate large quantities of cells quickly.
“We’re working on a disposable device where you wouldn’t even need a syringe pump to drive the separation,” says Rohit Karnik, Associate Professor of Mechanical Engineering at MIT. “You could potentially buy a $5 or $10 kit and get the cells sorted without needing any kind of [additional] instrument.”
While current cell-sorting technologies separate large batches of cells quickly and efficiently, they have several limitations.
Fluorescence-activated cell sorting, a widely used technique, requires lasers and voltage to sort cells based on their electric charge – a complex system requiring multiple parts. Researchers have also used fluorescent markers and magnetic beads that bind to desired cells, making them easy to spot and sift out. However, once collected, the cells need to be separated from the beads and markers – an added step that risks modifying the samples.
Going with the flow
Karnik’s team designed a compact cell sorter that requires no additional parts or steps. In concert with MIT’s Robert Langer and others, the team built upon their 2007 work in which they first came up with the sorting-by-rolling principle. The initial proof-of-principle design was relatively simple: Cells were injected into a single inlet, which gave way to a large chamber coated on one side with sticky, roll-inducing molecules. The incoming cells flowed through the chamber; the cells that bound to the molecules rolled to one side, then out to a collection chamber.
However, the researchers found that in order to allow target cells to first settle on the chamber’s surface, long channels were required, which would make the device too large. Instead, Choi came up with a surface pattern that causes cells to circulate within the chamber. The pattern comprises 10 parallel channels with 50 ridges and trenches, each ridge about 40 microns high. The researchers coated the ridges with P-selectin, a well-known molecule that promotes cell rolling. They then injected two kinds of leukemia cells: one with receptors for P-selectin, the other without.
They found that once injected, the cells entered the chamber and bounced across the top of the ridges, exiting the chip through an outlet. The cells with P-selectin receptors were “caught” by the sticky molecule and flipped into trenches that led to a separate receptacle. Through their experiments, the team successfully recovered the cells they intended to sift out with 96 percent purity.
Karnik says the device may be replicated and stacked to sort large batches of cells at relatively low cost. He and his colleagues are hoping to apply the device to sort other blood cells, as well as certain types of cancer cells for diagnostic applications and stem cells for therapeutic applications.In the future, Karnik envisions tailor-made cell rolling, designing molecules and surfaces that weakly adhere to any desired type of cell.
“It’s really the ability to design molecules to separate cells of interest that will be powerful,” Karnik says. “There’s no reason to believe it cannot be done, because nature has already done it.”
Read the published paper here: http://rsc.li/UkPpvd
Read the full MIT News article: http://bit.ly/12hAaYz