Inspired to Heal the Human Heart
DESIGN INSPIRATION OFTEN STRIKES ELLEN ROCHE IN HER MIT LAB. But it can also emerge while she’s with her children at the New England Aquarium, where soft creatures such as jellyfish share characteristics with the human heart. Or when she sees textiles with unique structures and weaves that could be used to reinforce devices that support and repair cardiac function.
The Latham Family Career Development Professor and an associate professor at MIT’s Institute for Medical Engineering and Science and in the Department of Mechanical Engineering, Roche takes such ideas back to her lab, where she and her team use combinations of organic tissue and synthetic, robotic components to design groundbreaking medical devices.
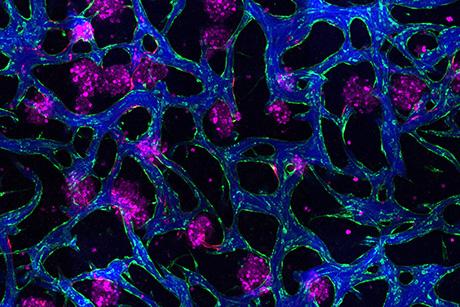
One device draws on the motion of jellyfish, combined with clinical imaging datasets, to replicate the diaphragm’s movement. Another helps the heart’s ventricles eject blood, using a motion akin to the simultaneous squeezing and wringing of a towel or sponge. The lab has also developed a new type of medical mesh, inspired by textiles and enabled by advancements in 3-D printing, that helps support and repair cardiac function.
“The challenge of integrating synthetic mechanical devices into a dynamic organ such as the heart, with the aim to repair or augment function, inspires me,” says Roche, leader of the Therapeutic and Technology Design and Development research group and recipient of the 2022 MIT Future Founders Prize Competition grand prize.
Roche’s approach to medical device design transcends the traditional boundaries of individual scientific fields. A bioengineer by training, Roche also attended medical school and went on to work with medical device companies Mednova Medical Technologies, Abbott Laboratories, and Medtronic, where she helped design blood filters, coronary stents, and a minimally invasive system for replacing aortic valves. She then completed her PhD at Harvard University and a postdoc in Ireland (her native country) before joining the faculty at MIT in 2017.
Her 25-person lab, working closely with industry partners and clinicians, focuses on designing minimally invasive devices to improve and support failing organs and deliver drugs. The lab also develops realistic benchtop models to test and monitor device effectiveness. Roche’s research recently expanded to include respiratory care and diabetes. But her primary focus remains the heart, which continually adapts even as it pumps 2,000 gallons of blood a day.
“Of course, with all this complexity, sometimes parts fail,” says Roche. “We design devices that can repair part of the function and integrate very seamlessly with the native heart function.”
Track record of innovation
Roche and her team use computational analysis, soft robotics, advanced materials, and evolving imaging techniques to understand how to help the heart without affecting its native function or leaving synthetic material behind.
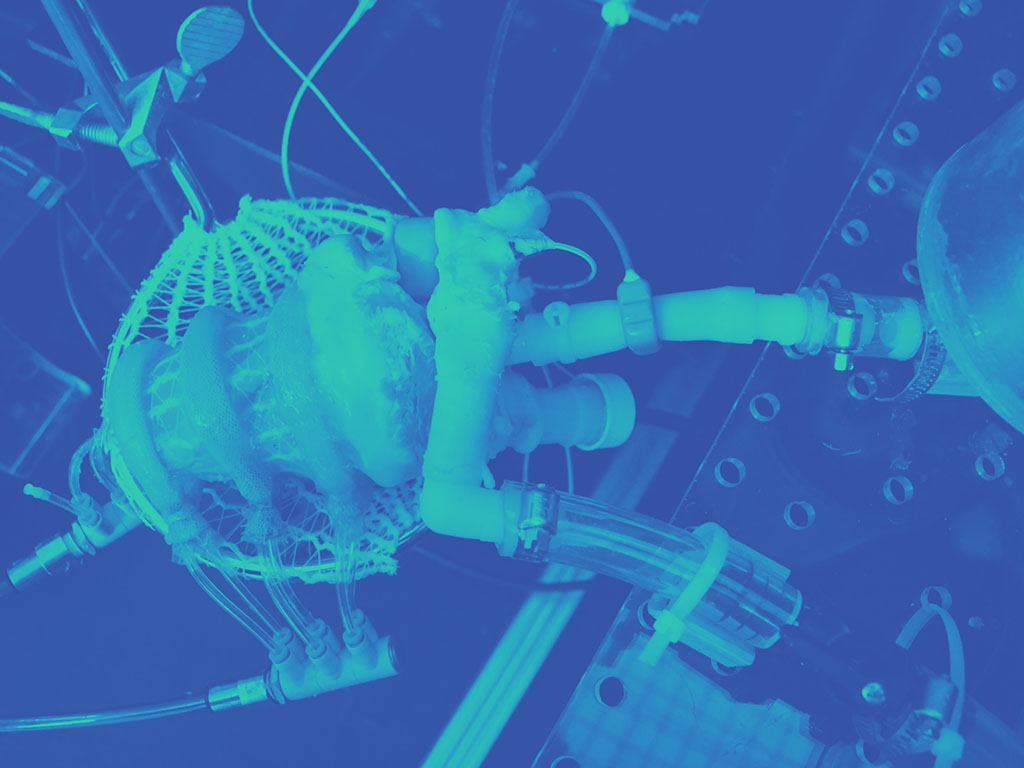
Their medical device designs include a biohybrid heart, which combines a pig heart and mechanical parts in a soft robotic matrix that replaces heart muscle and is bonded to inner heart tissue. The robotic muscles squeeze and twist the inner heart, mimicking the way a real human heart beats and pumps blood.
Roche and her team have used the biohybrid heart to simulate disease and then repair it with different types of valves. Designed to be a benchtop model (versus an implantable device), it provides an innovative way to better visualize and test repair options and to train interventionalists.
Roche’s lab also has developed:
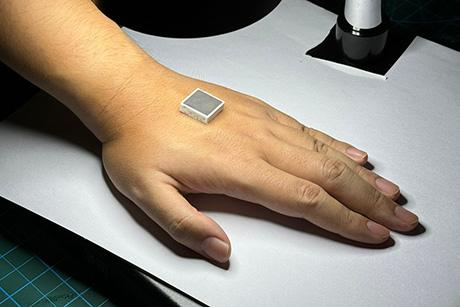
- A device that attaches directly to a damaged heart and delivers medicine to the area where it is most needed. This device has been modified to also deliver treatment for type 1 diabetes.
- A soft robotic sleeve that is implanted around the heart to support pumping, and recently a diaphragmatic assist device to rescue ventilation.
- A new catheter system that can affix a biodegradable patch to close a hole in the heart. Currently being commercialized by a company in Paris, the catheter is expected to be available for patient use in 2023.
The first two of these example designs feature biomaterials that don’t need to come in contact with blood, dramatically reducing the risks of infection and blood clots. Because most can be delivered minimally invasively, they ensure shorter procedures and hospital stays for the people who receive them.
Setting design requirements
To begin the design process for each project, Roche first assembles a multidisciplinary team that may include mechanical engineers, bioengineers, material scientists, pharmacists, surgeons, and other doctors. She typically involves clinicians from the get-go, so her team can establish functional requirements, parameters, and engineering specifications for a functional product that addresses a real need.
“Having that team from the beginning just really helps to accelerate the development and ensure that everyone’s on the same page,” she says. “It really helps with innovative, new ideas because often people with unique expertise or a slightly different background can bring practical knowledge and a fresh perspective.”
Roche’s strategy is to collaborate, design, iterate, test and ultimately simplify each design while keeping in mind the ultimate goal of making a difference in the lives of people. She says, “Whether you’re designing a medical device or a building or a structure, a lot of the same principles apply.”
In the long term, Roche dreams of creating a totally artificial soft robotic heart, a feat that would help address the long waiting list for heart transplants. “Ultimately, it would be amazing to build a completely functioning organ,” Roche says.
Article Source: MIT Spectrum
—Pamela Ferdinand
Pamela Ferdinand is a 2003–04 MIT Knight Science Journalism Fellow.